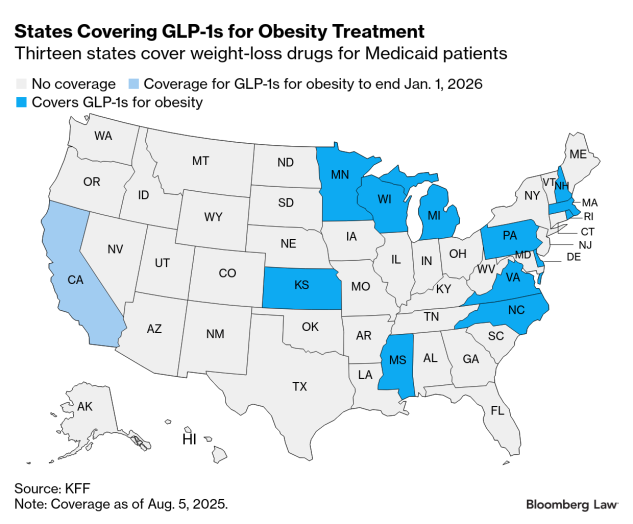
The Trump administration faces an arduous task of encouraging states to expand Medicaid coverage of expensive anti-obesity drugs as governments face strained budgets and sweeping federal cuts in the president’s tax-and-spending law.
A pilot project to be tested through the federal Center for Medicare and Medicaid Innovation would offer states options to experiment with coverage of GLP-1 drugs for obesity, people familiar with the matter told Bloomberg.
The plan, which hasn’t been formally announced by the Centers for Medicare & Medicaid Services, could encourage other states to join the 13 that offer Medicaid coverage for GLP-1s for obesity treatment. These drugs, including
But word of the pilot program comes as some states that currently cover the drugs are re-evaluating those decisions, citing high costs and budget shortfalls. California lawmakers voted in June to end Medicaid coverage of weight-loss drugs as part of a bid to reduce the state’s $12 billion budget deficit.
Health policy researchers say states that have yet to expand coverage are likely to remain hesitant as they grapple with the nearly $1 trillion in cuts to Medicaid in President Donald Trump’s recent tax law.
Policy analysts say the administration could include in its pilot program flexibility for states to implement cost containment strategies, including subscription agreements with manufacturers at negotiated prices. Giving states latitude in how they cover the drugs, analysts say, could help limit upfront costs and potentially promise states savings on medical care for heart disease, type 2 diabetes, and other weight-related conditions.
“It’s a question, of how quickly do savings elsewhere in the system accrue and do they do so at a quick enough pace to offset the upfront spending that might be associated with broader access to the products?” said Jack Rollins, director of federal policy at the National Association of Medicaid Directors.
States Under Pressure
As more states added anti-obesity drugs to their Medicaid coverage, spending and demand have rapidly surged, forcing states to weigh the sustainability of continuing to cover these drugs.
The number of anti-obesity medications reimbursed by state Medicaid programs increased by more than 7,600 in 2011 to roughly 108,000 in 2022, according to a 2024 report by researchers at Brigham and Women’s Hospital and Harvard Medical School. From 2022 to 2023, both Medicaid prescriptions and gross spending on GLP-1s nearly doubled, according to analysis from the health policy research and polling organization KFF.
Of the 13 states that cover the drugs for weight loss, most have utilization controls to limit eligibility for coverage, including prior authorization or BMI requirements.
“That’s a strategy for reducing the number of people that will be covered and arguably prioritizing the most affected population,” said Katherine Hempstead, a senior policy officer at the health policy philanthropy Robert Wood Johnson Foundation.
Even with those limits, some states are re-evaluating their coverage. California’s decision to stop covering obesity drugs in Medicaid is expected to save the state $85 million from 2025 to 2026 and an estimated $680 million annually by mid-2029.
Leaders in Connecticut, facing a $290 million Medicaid deficit, have moved away from an initial proposal to cover GLP-1s for obesity, and are now requiring patients to get a Type 2 diabetes diagnosis to qualify for coverage.
Due to the higher than anticipated costs, as well as the federal Medicaid cuts, “there’s going to be a lot of pressure on state budgets, and states are likely going to have to rethink expanding benefits,” said Liz Williams, senior policy manager at KFF’s Program on Medicaid and the Uninsured.
Cost Containment
Policy researchers say there are options the Trump administration could include in its pilot program to make GLP-1 coverage for obesity more financially sustainable for states and beneficial for patients’ long-term health.
The Trump administration’s reported plans for the pilot project, which come after it walked back a Biden-era proposal to cover the drugs, include requirements that Medicare plans and state Medicaid programs also provide patients with diet and exercise coaching, according to the Washington Post, which first reported the plans.
A CMS spokesperson declined to share additional details on the potential model but said in an emailed statement to Bloomberg Law that “all drug coverages undergo a cost-benefit review.”
Beyond healthy lifestyle support that can help ensure patients maintain the weight loss from using GLP-1 drugs, state Medicaid programs could also benefit from an option in the pilot program to opt for additional price negotiations with manufacturers or experiment with subscription models, said David Kim, an assistant professor of medicine and public health sciences at the University of Chicago.
A subscription model, in which states agree to cover at least a certain number of treatments at a price negotiated with the manufacturer, is the approach Louisiana adopted to pay for curative hepatitis C treatment for as many as 40,000 residents covered by Medicaid.
Another option is the use of value-based agreements, similar to the CMMI’s Cell and Gene Therapy Model in which 33 states are participating to increase access to treatments for sickle cell disease. Under the model, states agree to make the treatment accessible in exchange for supplemental rebates based on the drug’s performance.
Such models could help limit upfront costs to states while promoting “longer-term positive health outcomes and longer-term potential savings on chronic diseases,” Williams said.
To contact the reporter on this story:
To contact the editors responsible for this story:
Learn more about Bloomberg Law or Log In to keep reading:
See Breaking News in Context
Bloomberg Law provides trusted coverage of current events enhanced with legal analysis.
Already a subscriber?
Log in to keep reading or access research tools and resources.